Postingan Populer
How Much Vitamin C Do I Need For A Miscarriage
Layering Vitamin C And Retinol
Foods That Do Not Contain Vitamin C
Taking Vitamin D Supplements But Still Deficient - 15 Subtle Vitamin D Deficiency Symptoms That Most People ... : You should not take any vitamin d supplements without first having a blood test.
Is Vitamin C Good For The Lungs
- Review
- Open Access
- Published:
The effect of vitamin C on bronchoconstriction and respiratory symptoms caused by exercise: a review and statistical analysis
Allergy, Asthma & Clinical Immunology volume 10, Article number:58 (2014) Cite this article
-
20k Accesses
-
9 Citations
-
84 Altmetric
-
Metrics details
Abstract
Physical activity increases oxidative stress and therefore the antioxidant effects of vitamin C administration might become evident in people undertaking vigorous exercise. Vitamin C is involved in the metabolism of histamine, prostaglandins, and cysteinyl leukotrienes, all of which appear to be mediators in the pathogenesis of exercise-induced bronchoconstriction (EIB). Three studies assessing the effect of vitamin C on patients with EIB were subjected to a meta-analysis and revealed that vitamin C reduced postexercise FEV1 decline by 48% (95% CI: 33% to 64%). The correlation between postexercise FEV1 decline and respiratory symptoms associated with exercise is poor, yet symptoms are the most relevant to patients. Five other studies examined subjects who were under short-term, heavy physical stress and revealed that vitamin C reduced the incidence of respiratory symptoms by 52% (95% CI: 36% to 65%). Another trial reported that vitamin C halved the duration of the respiratory symptoms in male adolescent competitive swimmers. Although FEV1 is the standard outcome for assessing EIB, other outcomes may provide additional information. In particular, the mean postexercise decline of FEF50 is twice the decline of FEV1. Schachter and Schlesinger (1982) reported the effect of vitamin C on exercise-induced FEF60 levels in 12 patients suffering from EIB and their data are analyzed in this paper. The postexercise FEF60 decline was greater than 60% for five participants and such a dramatic decline indicates that the absolute postexercise FEF60 level becomes an important outcome in its own right. Vitamin C increased postexercise FEF60 levels by 50% to 150% in those five participants, but had no significant effect in the other seven participants. Thus, future research on the effects of vitamin C on EIB should not be restricted to measuring only FEV1. Vitamin C is inexpensive and safe, and further study on those people who have EIB or respiratory symptoms associated with exercise is warranted.
Introduction
Exercise-induced bronchoconstriction (EIB) describes the acute narrowing of the airways that occurs as a result of vigorous exercise [1–3]. The emergence of EIB depends on the kind and level of physical activity, and also on the humidity and temperature of the inhaled air [1–3].
Only about 10% of the non-asthmatic general population suffer from EIB, whereas up to 90% of asthmatics may suffer from EIB [2–4]. Thus EIB is a common phenotype of asthma. EIB is also common among competitive athletes even if they do not have asthma, and it is particularly prevalent in endurance sports, such as running, winter sports and swimming [2, 3, 5].
Usually, a decline of 10% or greater in FEV1 after exercise is classified as EIB, but other cut off limits have also been used [1–3]. However, EIB is not an arbitrary dichotomous condition, instead there is a continuous variation in the possible level of FEV1 decline such that the 9% and 11% decline levels in FEV1 are not biologically different phenomena, although they fall on either side of the usual cut off level. A single constant percentage point cut off limit is thus simplistic. It is more useful to analyze the phenomenon as a continuous variable rather than a dichotomous variable. This issue is relevant when planning appropriate statistical analysis of outcomes related to EIB.
Symptoms are much more important than laboratory values for patients. However, the correlation between the declines in postexercise FEV1 values and postexercise respiratory symptoms is poor [4, 6, 7]. Therefore, respiratory symptoms should be recorded concurrently with the pulmonary function tests.
The stimulus for EIB seems to be the loss of water caused by increased ventilation. This leads to the release of mediators such as histamine, prostaglandins and leukotrienes, all of which cause bronchoconstriction [1–3, 8]. Nitric oxide also plays a role in the pathogenesis of EIB [9, 10]. Finally, oxidative stress seems to play role in the emergence of EIB [11, 12].
Vitamin C: exercise and airways
Physical activity increases oxidative stress [13], and therefore, as an antioxidant vitamin C might have particularly evident effects in people who are participating in vigorous exercise. Electron spin resonance studies have shown that vitamin C administration decreased the levels of free radicals generated during exercise [14, 15] and vitamin C administration attenuated the increases in oxidative stress markers caused by exercise [16–18].
The level of vitamin C in the lungs is high [19], and vitamin C levels in alveolar macrophages and alveolar type II cells are 30 times higher than in plasma [20]. About 10% of vitamin C in the lungs of rats is in a lavageable form [21], but the level of vitamin C in the bronchoalveolar lavage seems to be lower in humans than in rats [22]. In any case, high levels of vitamin C in the lungs imply that the vitamin may protect the lungs against oxidative stress.
Unlike guinea pigs and humans, mice and rats are able to synthesize vitamin C, and these species are able to increase their rates of vitamin C synthesis under certain stressful conditions. Ozone exposure in rats and mice significantly increased vitamin C levels in bronchoalveolar lavage fluid [23, 24], which might serve as a protective response to the higher oxidative stress level being encountered. Exposure to ozone and nitrogen dioxide decreased lung vitamin C levels in guinea pigs, which implies that the vitamin was consumed while it protected against the oxidants [25, 26]. Vitamin C administration in guinea pigs decreased mortality caused by ozone exposure [27, 28] and vitamin C deficiency in guinea pigs increased necrotic injury to type II lung cells upon H2O2 treatment [29]. Exposure to ozone in humans decreased the vitamin C level in the respiratory tract lining fluid [30]. Thus, given that oxidative stress seems to play a role in EIB [11, 12], vitamin C might protect against EIB through non-specific antioxidant effects. Nevertheless, there are also more specific biochemical mechanisms through which vitamin C may influence pulmonary functions.
Histamine is one of the mediators involved in the pathogenesis of EIB [1–3, 8]. It is released from mast cells, which have a high concentration of vitamin C [31]. Furthermore, the release of histamine causes oxidation of vitamin C in the mast cells [32]. In guinea pigs, a deficiency of vitamin C increased histamine levels in their plasma, urine and lungs [33, 34], whereas a high dosage of vitamin C decreased their plasma histamine levels [35]. In vitamin C deficient guinea pigs, a single dose of vitamin C rapidly decreased plasma and urine histamine levels to normal levels [34]. In rats, vitamin C attenuated the increases in histamine levels, which were caused by various stressful conditions including cold and heat stress [36]. Four trials conducted on humans found that the administration of vitamin C significantly decreased plasma histamine levels [37–40]. Vitamin C decreased bronchoconstriction caused by histamine in living guinea pigs [33, 41–44], and it decreased contractions caused by histamine in isolated guinea pig trachea smooth muscle [45, 46]. Finally, in guinea pigs exposed to ozone, vitamin C decreased bronchial reactivity to histamine [47].
Prostaglandins (PGs) and leukotrienes (LTs) also participate in the pathogenesis of EIB [1–3, 8]. Vitamin C deficiency in guinea pigs increased the level of bronchoconstrictor PGF2α in the trachea [44, 48], and increased the in vitro synthesis of PGF2α in lung microsomes [49]. Vitamin C deficiency decreased the production of PGE2 in guinea pig trachea [48]; PGE2 causes smooth muscle relaxation and may protect against EIB [1, 2]. Furthermore, hyper-responsiveness to histamine in vitamin C deficient guinea pigs was further increased by indomethacin [44], and the relaxing effects of vitamin C on isolated guinea pig trachea were inhibited by indomethacin [46]. Indomethacin also blocked the effect of vitamin C on methacholine-induced bronchoconstriction in humans [50]. The influence of indomethacin on vitamin C effects is a further indication that the pulmonary effects of vitamin C may be partly mediated through the influences of vitamin C on the PG metabolism. Furthermore, vitamin C decreased contractions caused by PGF2α in guinea pig tracheal tube preparations [48]. Finally, the administration of vitamin C in humans reduced the postexercise increase in the urinary markers of bronchoconstrictors PGD2 and cysteinyl LTs [51].
Nitric oxide (NO) has also been implicated in the pathogenesis of EIB [9, 10]. The metabolism of NO is altered in EIB patients but it is not correlated with exercise-induced changes in spirometry [9]. Vitamin C was reported to decrease the NO level in EIB patients [51].
A single oral dose of vitamin C can rapidly elevate mucosal vitamin C levels. Nasal lavage fluid vitamin C levels in human subjects increased by three-fold in two hours after a single dose of 1 or 2 g of vitamin C [52, 53]. The rapid transport of ingested vitamin C to the respiratory tract lining fluid implies that even single doses of vitamin C might be effective in protecting against acute increases in oxidative stress in the airways.
FEV1 decline caused by exercise
Three randomized, double-blind, placebo-controlled cross-over trials examined the effect of vitamin C (0.5 to 2 g/day) on exercise-induced FEV1 decline (Table 1). The pooled effect of vitamin C (Figure 1) indicates a reduction in the postexercise FEV1 decline of 48% (95% CI: 33% to 64%) [54, 55]. In one study, Tecklenburg et al. reported that the postexercise FEV1 decline was 12.9% after the placebo period, but only 6.4% after a 2-week vitamin C administration, which corresponds to 50% reduction in the postexercise FEV1 decline [51] (Figure 1). In other two studies, vitamin C was administered as a single dose 1 or 1.5 hours before the exercise test [56, 57], yet the effects were the same as for the first study (Figure 1). Thus, a single dose of vitamin C before an exercise session appears to be sufficient to generate the same benefit as a 2-week supplementation regime. This may be explained by the rapid transfer of vitamin C to the airway lining fluids [52, 53].
Full size table
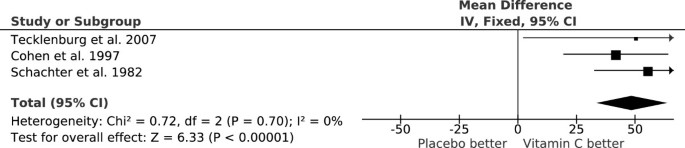
Reduction of postexercise FEV 1 decline by vitamin C. The vertical lines indicate the 95% CI for the three trials that studied the effect of vitamin C administration on EIB and the squares in the middle of the lines indicate the point estimates of the studies. An effect of 100% would indicate full prevention of postexercise FEV1 decline. The diamond shape at the foot indicates the 95% CI for the pooled vitamin C effect: 48% (95% CI: 33% to 64%) reduction in postexercise FEV1 decline. For example, Tecklenburg et al. reported a 12.9% FEV1 decline after placebo, but only a 6.4% decline after the vitamin C period, which corresponds to a 50% reduction in FEV1 decline by vitamin C [51]. This figure is based on data published in [54].
Full size image
The three EIB trials included a total of only 40 participants. However, the trials were carried out over three different decades and on two different continents. The criteria for EIB differed and the mean ages of the participants were 14 years in one study [57] but 25 and 26 years in the two other studies [51, 56]. Nevertheless, all the studies are consistent with vitamin C halving the postexercise FEV1 decline (Figure 1). It is not clear how far this estimate can be generalized, but similar findings from such dissimilar studies indicate that vitamin C may be effective for a wider population who suffer from EIB.
A fourth randomized cross-over trial on 8 participants who suffered from EIB found that the combination of 0.5 g/day of vitamin C along with vitamin E significantly decreased the FEV1 decline at 5, 15 and 30 min after exercise [58]. Although this finding is not specific to vitamin C, it is consistent with the benefits of antioxidants. In 5 other participants who did not suffer from EIB, postexercise FEV1 decline was not influenced by the combination of the vitamins [58]. This study was published only as an abstract.
FEF60 and PEF declines caused by exercise
FEV1 is the standard outcome for assessing whether a patient suffers from EIB [1–3]. However, exercise-induced decline in FEF25-75 is twice as great as the decline in FEV1, and therefore, FEF25-75 may provide relevant information in addition to FEV1 data [6, 7, 9, 10, 59–63]. Furthermore, FEF50 values essentially give the same information as FEF25-75[64].
Schachter and Schlesinger (1982) studied 12 participants with EIB and published the effects of a single dose of 0.5 g vitamin C on the FEV1, PEF, and FEF60 levels on each participant before and after the exercise test [56]. Figure 2 shows the data for 5 minutes after the exercise test. As a pulmonary function measure, the FEF60 level is close to the FEF50 level. On the basis of the slope of the linear regression line (Figure 2A), vitamin C decreased the postexercise FEV1 decline by 55% (95% CI: 32% to 78%). The mean decline in postexercise FEV1 was 18% on the placebo day. The greatest postexercise FEV1 decline on the placebo-day was 52% in participant #11 (Figure 2A).
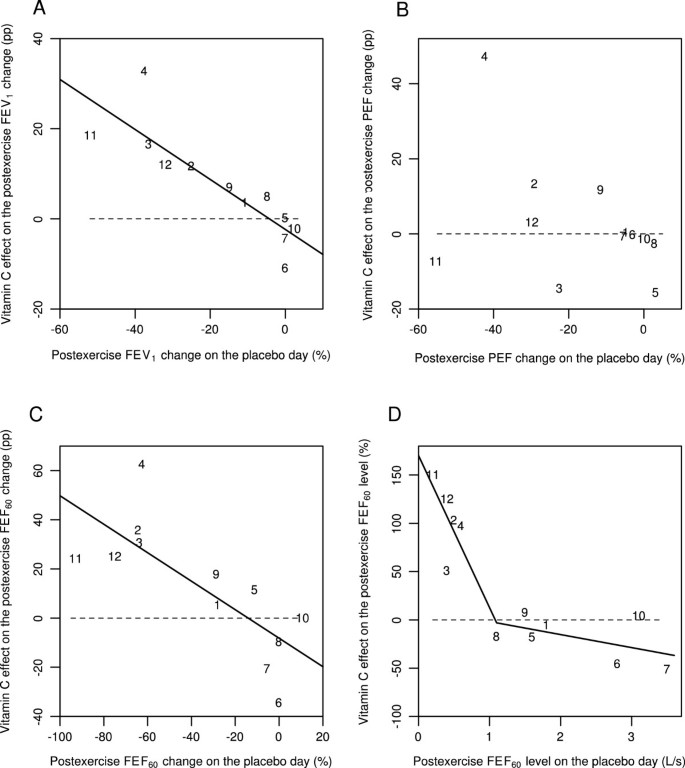
Effect of vitamin C on 5-min postexercise changes for different pulmonary function outcomes according to the study by Schlesinger and Schachter [56]. Effect of vitamin C on the following 5 min after exercise: A) the postexercise FEV1 change, B) the postexercise PEF change, C) the postexercise FEF60 change, and D) the postexercise FEF60 level. Figures 2A, B, and C show the effect of vitamin C in percentage points (pp). For example, on the placebo-day, participant #11 had a postexercise FEV1 decline of 52%, and on the vitamin C day a postexercise FEV1 decline of 33%, which gives the 19 pp improvement shown in Figure 2A. Figure 2D shows the effect of vitamin C in percentages. For example, on the placebo-day, participant #11 had a postexercise FEF60 level of 0.2 L/s, and on the vitamin C day a postexercise FEF60 level of 0.5 L/s, which gives the 150% increase shown in Figure 2D. Figure 2 uses the same identification numbers for participants as those used in the original paper [56]. The dash lines indicate equality between vitamin C and placebo. If vitamin C had no effect, the observations would be located randomly and symmetrically around the dash lines. The continuous lines indicate the regression lines. In Figure 2A, the addition of the placebo-day postexercise FEV1 change to the model containing the intercept improved the model fit by χ2(1 df) =16.5 (P =0.0001). In Figure 2B, the slope did not significantly differ from the null effect and therefore the regression line is not shown. In Figure 2C, addition of the placebo-day postexercise FEF60 change to the model containing the intercept improved the model fit by χ2(1 df) =10.5 (P =0.001). In Figure 2D, adding the two spline segments with the knot at 1.1 L/s to the model containing only the intercept improved the model fit by χ2(2 df) = 24.7 (P = 0.000004). For the statistical methods of Figure 2, see Additional file 1.
Full size image
PEF is not recommended for assessing EIB, since it is less repeatable than FEV1[2, 3]. The study by Schachter and Schlesinger found that vitamin C had no consistent effect on postexercise PEF decline (Figure 2B). The linear regression slope did not significantly differ from the null effect, thus it is not shown. However, all of the 5 participants who did benefit of vitamin C as measured by its effects on FEV1, also had a substantial postexercise PEF decline of 20% or more on the placebo day. Thus, a high PEF decline on the placebo day identified all the 5 participants who benefited from vitamin C as evaluated by FEV1. This finding might have practical importance since PEF measurements are much easier to carry out than spirometry.
The linear regression slope indicated that vitamin C decreased the postexercise FEF60 decline by 58% (95% CI: 23% to 92%) (Figure 2C). The mean decline in postexercise FEF60 was 35% on the placebo day, which is twice the decline in FEV1 (18%). The ratio of about two for the declines in FEF25-75 and FEV1 has been reported previously [6, 7, 9, 10, 62, 63]. The exercise-induced FEF60 decline on the placebo day was greater than 60% in 5 out of the 12 participants, with the greatest decline being 92% for participant #11 (Figure 2C).
A dramatic postexercise FEF60 decline in 5 participants indicates that the absolute postexercise level of FEF60 becomes an important outcome in its own right. For each of these 5 participants, the postexercise FEF60 level was less than 1 L/s on the placebo day (Figure 2D). Moreover, vitamin C administration increased the postexercise FEF60 level in these 5 participants by between 50% and 150%. In contrast, no mean difference between the vitamin C and placebo days was detected in the other 7 participants. This indicates that the effect of vitamin C may be restricted to those EIB patients who had postexercise FEF60 levels below 1 L/s.
Schachter and Schlesinger also reported the FEF60(P) values, which were based on partial flow volume curves [56]. The effect of vitamin C on FEF60(P) was similar to its effects on FEF60. This analysis is shown in Additional file 1.
Finally, Schachter and Schlesinger reported the FEV1, PEF, FEF60 and FEF60(P) values also for the time point immediately after the exercise (0 min) [56]. At the 0 minute data, vitamin C and placebo days differ significantly when analyzed by linear regression (Figure 3). On the basis of the slopes, vitamin C decreased the postexercise FEV1 changes by 86% (95% CI: 24% to 147%), postexercise PEF changes by 74% (95% CI: 31% to 118%), and postexercise FEF60 changes by 90% (95% CI: 9% to 171%). Postexercise FEF60 levels were also significantly influenced by vitamin C (P =0.003). Thus, significant effects on postexercise pulmonary function changes by vitamin C can also be seen immediately after the exercise challenge test. This analysis is shown in Additional file 2.
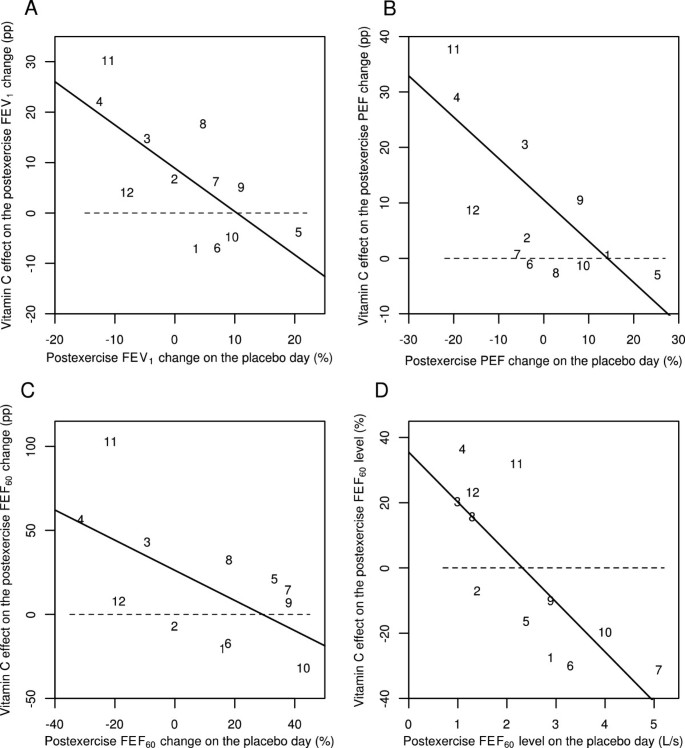
Effect of vitamin C on 0-min postexercise changes for different pulmonary function outcomes according to the study by Schlesinger and Schachter [56]. Effect of vitamin C on the following immediately (0 min) after exercise: A) the postexercise FEV1 change, B) the postexercise PEF change, C) the postexercise FEF60 change, and D) the postexercise FEF60 level. Figures 3A, B, and C show the effect of vitamin C in percentage points (pp). For example, on the placebo-day, participant #11 had a postexercise FEV1 change of -11%, and on the vitamin C day a postexercise FEV1 change of +19%, which gives the 30 pp improvement shown in Figure 3A. Figure 3D shows the effect of vitamin C in percentages. For example, on the placebo-day, participant #11 had a postexercise FEF60 level of 2.2 L/s, and on the vitamin C day a postexercise FEF60 level of 2.9 L/s, which gives the 32% increase shown in Figure 3D. Figure 3 uses the same identification numbers for participants as those used in the original paper [56]. The dash lines indicate equality between vitamin C and placebo. If vitamin C had no effect, the observations would be located randomly and symmetrically around the dash lines. The continuous lines indicate the regression lines. In Figure 3A, addition of the placebo-day postexercise FEV1 change to the model containing the intercept improved the model fit by χ2(1 df) =8.2 (P =0.004). In Figure 3B, addition of the placebo-day postexercise PEF change to the model containing the intercept improved the model fit by χ2(1 df) =10.8 (P =0.001). In Figure 3C, addition of the placebo-day postexercise FEF60 change to the model containing the intercept improved the model fit by χ2(1 df) =5.7 (P =0.02) and in Figure 3D, addition of the placebo-day postexercise FEF60 level to the model containing the intercept improved the model fit by χ2(1 df) =11.4 (P =0.001). For the statistical methods of Figure 3, see Additional file 2.
Full size image
Respiratory symptoms caused by exercise
In evidence-based medicine (EBM) the primary focus of interest is on clinically relevant outcomes, including symptoms such as cough, sore throat and dyspnea. From the EBM perspective, laboratory outcomes such as FEV1 are surrogates and of secondary importance. Postexercise FEV1 decline and postexercise respiratory symptoms are poorly correlated [4, 6, 7]. Therefore, the effects of vitamin C administration on respiratory symptoms caused by exercise is an important question that should be concurrently considered along with its effects on the pulmonary function test values. None of the three trials on vitamin C and EIB recorded respiratory symptoms associated with the exercise tests (Table 1). Nevertheless, Tecklenburg et al. did report that asthma symptoms were less intense after the vitamin C administration period compared with the placebo period [51].
Five separate randomized placebo-controlled trials reported the effects of vitamin C on respiratory symptoms during and after heavy physical stress and, according to a meta-analysis, vitamin C administration decreased the incidence of respiratory symptoms in these studies by 52% (95% CI: 36% to 65%) [65, 66]. Three of the studies were conducted on marathon runners [67–69], one study used Canadian soldiers on a winter exercise [70], and one study was on schoolchildren in a skiing camp in the Swiss Alps [71]. In the general population, acute cough and sore throat usually indicates a viral etiology, and authors of these five trials assumed that the respiratory symptoms were caused by viruses. However, when such symptoms occur after a marathon run and other endurance sports sessions, they are not always caused by a viral infection. Instead they can result from an injury to the airway epithelium caused by hours of exceptional ventilatory exertion [1–3, 72]. Respiratory symptoms associated with physical irritation of the airways, allergy, and viral infections are all similar [66, 73], and therefore there is no justification to assume that all the respiratory symptoms in the above-mentioned five studies were caused by viruses. In one study, 70% of respiratory symptom episodes of elite athletes were caused by a non-infectious etiology, which indicates high prevalence of respiratory symptoms not caused by viruses in athletes [74].
In their study on marathon runners, Peters et al. [67] recorded the "self-reported symptoms including a running nose, sneezing, sore throat, cough" during a 2-week period after the race [67]. The incidence of post-race cough was reduced by 71% in the vitamin C group as compared to the placebo group (P[2-t] =0.014; 4/43 vs. 13/41). The incidence of sore throat was reduced by 67% in the vitamin C group (P =0.001; 8/43 vs. 23/41). In contrast, vitamin C had no significant effect on the incidence of runny nose (P =0.2; 13/43 vs. 19/41), which is the most bothersome symptom of rhinovirus infections [75]. Peters et al. did not carry out any virologic or pulmonary function tests and therefore the etiology of the cough and sore throat in the marathon runners is uncertain [67]. It is plausible that the common cold studies on marathon runners may have been partly measuring the effect of vitamin C on the epithelial injury caused by the heavy exertional ventilation.
A recent randomized trial in Israel found that vitamin C shortened the duration of respiratory symptoms in male adolescent competitive swimmers by 47% (95% CI: 14% to 80%), but no effect was seen in their female counterparts [76]. The difference found between the sexes in that study was significant (P =0.003). Here too, the etiology was not investigated and the respiratory symptoms might have been partly caused by the non-infectious irritation of swimmers' airways [77].
Thus, in six randomized trials vitamin C reduced the incidence and duration of respiratory symptoms caused by heavy physical activity, yet it is not clear to what extent the symptoms were caused by non-infectious injury to the airway epithelium as opposed to viral infections. Irrespective of the etiology, symptoms are more important from the EBM point of view than laboratory measurements such as FEV1.
Asthma phenotypes other than EIB
Asthma is a heterogeneous syndrome, an "umbrella concept," that includes different phenotypes with different underlying pathophysiologies [78, 79]. Therefore, it is relevant to consider whether the effects of vitamin C are limited to EIB or whether vitamin C might also influence other asthma phenotypes. There is evidence that some virus infections cause a transient increase in oxidative stress [80, 81], and a systematic review identified three studies that indicated that vitamin C might protect against common cold-induced asthma [82].
A 4-month trial on 154 British asthmatics showed that the FEV1 level was not influenced by 1 g/day of vitamin C [83]. Moreover, the FEV1 level was not influenced by a 5-year administration of 0.25 g/day of vitamin C along with vitamin E and β-carotene in a large-scale trial on 20,536 British adults [84]. These two British-based studies imply that vitamin C supplementation does not influence pulmonary functions in patients with stable asthma or in relatively healthy people. Nevertheless, the study on British asthmatics found that the need for inhaled corticosteroids was lower in the vitamin C group [85]. In any case, vitamin C may beneficially influence pulmonary functions of some people under certain forms of acute stress, such as when they endure heavy physical activity or suffer from a viral respiratory tract infection.
Refocusing research onto the biological effects of vitamin C
Vitamin C was identified in the search for the substance the deficiency of which led to scurvy. These early studies led to the assumption that the sole physiological function of vitamin C is just to prevent and treat scurvy. Therefore, it is often assumed that higher doses of vitamin C have no benefit when a person does not suffer from scurvy. In view of this strongly entrenched assumption, assessing the role of vitamin C on diseases and conditions other than scurvy is not just an empirical question but also a conceptual issue.
Bias against vitamin supplementation in general has been well documented [86–88]. Several influential reviews on vitamin C and the common cold have been shown to be erroneous and misleading [89, 90]. A Cochrane review on vitamin C and asthma [91] was shown to have substantial errors in the extraction of data and data analysis, and the review misled readers for a decade [92, 93]. If we wish for progress in the understanding of the effects of vitamin C on EIB, it needs to be acknowledged that the effects of vitamin C are not limited to the prevention of scurvy alone and, consequently, the published data on vitamin C should be analyzed carefully and comprehensively.
Conclusions
Three trials have examined the effect of vitamin C on EIB and found that 0.5 to 2 g/day vitamin C halved the postexercise FEV1 decline (Figure 1). In addition, five trials found that vitamin C administration halved the incidence of respiratory symptoms after short-term heavy physical stress [65, 66] and one other trial found that vitamin C administration halved the duration of the respiratory symptoms in male adolescent competitive swimmers [76]. Although these nine randomized trials indicate that vitamin C has genuine biological effects on pulmonary functions and respiratory symptoms in people doing heavy exercise, it is unclear how the effect of vitamin C depends on the kind of physical activity, the level of physical activity, the temperature of ambient air, the humidity of ambient air, the dose of vitamin C, the level of dietary intake of vitamin C, and various other factors.
Given the safety and low cost of vitamin C, and the consistency of positive findings in the nine randomized trials on vitamin C against EIB and respiratory symptoms, it seems reasonable for physically active people to test whether vitamin C is beneficial on an individual basis, if they have documented EIB or suffer from respiratory symptoms such as cough or sore throat after taking vigorous exercise.
In future studies on vitamin C and EIB, a variety of pulmonary function outcomes should be measured. Although FEV1 is currently the standard measure for diagnosing EIB, the effect of vitamin C on the postexercise FEF50 and FEF75 levels should also be examined, as they may provide important additional information (Figures 2D and 3D). Furthermore, PEF is much easier to measure than carrying out a spirometry test. Therefore the possibility that the postexercise PEF decline might identify those people who may benefit from vitamin C administration should be investigated (Figures 2B and 3B).
In the earlier studies on vitamin C and EIB, only the mean effects on FEV1 were calculated [51, 56] or EIB was dichotomized by an arbitrary cut off level [57]. However, postexercise FEV1 decline is a continuous variable and the effect of vitamin C may depend on the baseline level of pulmonary function decline. Therefore, linear modelling is a much more informative way to analyze vitamin C effects on pulmonary functions and it should be used in the future studies (Figures 2 and 3). Finally, while pulmonary function tests are objective outcomes, symptoms are much more relevant outcomes from the EBM point of view, therefore both of them should be recorded concurrently in future investigations.
Abbreviations
- CI:
-
Confidence interval
- EBM:
-
Evidence based medicine
- EIB:
-
Exercise-induced bronchoconstriction
- FEF25-75:
-
Mean forced expiratory flow between 25% and 75% of FVC
- FEF50:
-
Forced expiratory flow when 50% of the FVC has been expired
- FEF60:
-
Forced expiratory flow when 60% of the FVC has been expired
- FEF60(P):
-
FEF60 value based on partial flow volume curves
- FEF75:
-
Forced expiratory flow when 75% of the FVC has been expired
- FEV1:
-
Forced expiratory volume in 1 second
- FVC:
-
Forced vital capacity
- LT:
-
Leukotriene
- PEF:
-
Peak expiratory flow
- PG:
-
Prostaglandin.
References
- 1.
Anderson SD, Kippelen P: Airway injury as a mechanism for exercise-induced bronchoconstriction in elite athletes. J Allergy Clin Immunol. 2008, 122: 225-235. 10.1016/j.jaci.2008.05.001. http://www.ncbi.nlm.nih.gov/pubmed/18554705. http://dx.doi.org/10.1016/j.jaci.2008.05.001
Article PubMed Google Scholar
- 2.
Weiler JM, Anderson SD, Randolph C: Pathogenesis, prevalence, diagnosis, and management of exercise-induced bronchoconstriction. Ann Allergy Asthma Immunol. 2010, 105 (6): S1-S47. 10.1016/j.anai.2010.09.021.http://dx.doi.org/10.1016/j.anai.2010.09.021,
Article PubMed Google Scholar
- 3.
Parsons JP, Hallstrand TS, Mastronarde JG, Kaminsky DA, Rundell KW, Hull JH, Storms WW, Weiler JM, Cheek FM, Wilson KC, Anderson SD: An official American thoracic society clinical practice guideline: exercise-induced bronchoconstriction. Am J Respir Crit Care Med. 2013, 187: 1016-1027. 10.1164/rccm.201303-0437ST. http://www.ncbi.nlm.nih.gov/pubmed/23634861. http://dx.doi.org/10.1164/rccm.201303-0437ST
CAS Article PubMed Google Scholar
- 4.
De Baets F, Bodart E, Dramaix-Wilmet M, Van Daele S, de Bilderling G, Masset S, Vermeire P, Michel O: Exercise-induced respiratory symptoms are poor predictors of bronchoconstriction. Pediatr Pulmonol. 2005, 39: 301-305. 10.1002/ppul.20185. http://www.ncbi.nlm.nih.gov/pubmed/15678500. http://dx.doi.org/10.1002/ppul.20185
Article PubMed Google Scholar
- 5.
Thomas S, Wolfarth B, Wittmer C, Nowak D, Radon K, GA2LEN-Olympic study-Team: Self-reported asthma and allergies in top athletes compared to the general population - results of the German part of the GA2LEN-Olympic study 2008. Allergy Asthma Clin Immunol. 2010, 6: 31-10.1186/1710-1492-6-31.http://dx.doi.org/10.1186/1710-1492-6-31,
PubMed Central Article PubMed Google Scholar
- 6.
Rundell KW, Wilber RL, Szmedra L, Jenkinson DM, Mayers LB, Im J: Exercise-induced asthma screening of elite athletes: field versus laboratory exercise challenge. Med Sci Sports Exerc. 2000, 32: 309-316. 10.1097/00005768-200002000-00010. http://www.ncbi.nlm.nih.gov/pubmed/10694112. http://dx.doi.org/10.1097/00005768-200002000-00010
CAS Article PubMed Google Scholar
- 7.
Rundell KW, Im J, Mayers LB, Wilber RL, Szmedra L, Schmitz HR: Self-reported symptoms and exercise-induced asthma in the elite athlete. Med Sci Sports Exerc. 2001, 33: 208-213. http://www.ncbi.nlm.nih.gov/pubmed/11224807. http://dx.doi.org/10.1097/00005768-200102000-00006
CAS Article PubMed Google Scholar
- 8.
Hallstrand TS, Moody MW, Wurfel MM, Schwartz LB, Henderson WR, Aitken ML: Inflammatory basis of exercise-induced bronchoconstriction. Am J Respir Crit Care Med. 2005, 172: 679-686. 10.1164/rccm.200412-1667OC. http://www.ncbi.nlm.nih.gov/pubmed/15947280. http://dx.doi.org/10.1164/rccm.200412-1667OC
PubMed Central Article PubMed Google Scholar
- 9.
Shin HW, Schwindt CD, Aledia AS, Rose-Gottron CM, Larson JK, Newcomb RL, Cooper DM, George SC: Exercise-induced bronchoconstriction alters airway nitric oxide exchange in a pattern distinct from spirometry. Am J Physiol Regul Integr Comp Physiol. 2006, 291: R1741-R1748. 10.1152/ajpregu.00178.2006. http://www.ncbi.nlm.nih.gov/pubmed/16840654. http://dx.doi.org/10.1152/ajpregu.00178.2006
CAS Article PubMed Google Scholar
- 10.
Chinellato I, Piazza M, Peroni D, Sandri M, Chiorazzo F, Boner AL, Piacentini G: Bronchial and alveolar nitric oxide in exercise-induced bronchoconstriction in asthmatic children. Clin Exp Allergy. 2012, 42: 1190-1196. 10.1111/j.1365-2222.2012.03973.x. http://www.ncbi.nlm.nih.gov/pubmed/22805466. http://dx.doi.org/10.1111/j.1365-2222.2012.03973.x
CAS Article PubMed Google Scholar
- 11.
Baumann JM, Rundell KW, Evans TM, Levine AM: Effects of cysteine donor supplementation on exercise-induced bronchoconstriction. Med Sci Sports Exerc. 2005, 37: 1468-1473. 10.1249/01.mss.0000177479.57468.15. http://www.ncbi.nlm.nih.gov/pubmed/16177596. http://dx.doi.org/10.1249/01.mss.0000177479.57468.15
CAS Article PubMed Google Scholar
- 12.
Barreto M, Villa MP, Olita C, Martella S, Ciabattoni G, Montuschi P: 8-Isoprostane in exhaled breath condensate and exercise-induced bronchoconstriction in asthmatic children and adolescents. Chest. 2009, 135: 66-73. 10.1378/chest.08-0722.http://dx.doi.org/10.1378/chest.08-0722,
Article PubMed Google Scholar
- 13.
Powers SK, Nelson WB, Hudson MB: Exercise-induced oxidative stress in humans: cause and consequences. Free Radic Biol Med. 2011, 51: 942-950. 10.1016/j.freeradbiomed.2010.12.009. http://www.ncbi.nlm.nih.gov/pubmed/21167935. http://dx.doi.org/10.1016/j.freeradbiomed.2010.12.009
CAS Article PubMed Google Scholar
- 14.
Ashton T, Young IS, Peters JR, Jones E, Jackson SK, Davies B, Rowlands CC: Electron spin resonance spectroscopy, exercise, and oxidative stress: an ascorbic acid intervention study. J Appl Physiol. 1999, 87: 2032-2036.http://www.ncbi.nlm.nih.gov/pubmed/10601146,
CAS PubMed Google Scholar
- 15.
Davison GW, Ashton T, Davies B, Bailey DM: In vitro electron paramagnetic resonance characterization of free radicals: relevance to exercise-induced lipid peroxidation and implications of ascorbate prophylaxis. Free Radic Res. 2008, 42: 379-386. 10.1080/10715760801976618.http://dx.doi.org/10.1080/10715760801976618,
CAS Article PubMed Google Scholar
- 16.
Sánchez-Quesada JL, Jorba O, Payés A, Otal C, Serra-Grima R, González-Sastre F, Ordóñez-Llanos J: Ascorbic acid inhibits the increase in low-density lipoprotein (LDL) susceptibility to oxidation and the proportion of electronegative LDL induced by intense aerobic exercise. Coron Artery Dis. 1998, 9: 249-255. 10.1097/00019501-199809050-00002. http://www.ncbi.nlm.nih.gov/pubmed/9710684. http://dx.doi.org/10.1097/00019501-199809050-00002
Article PubMed Google Scholar
- 17.
Silvestro A, Scopacasa F, Oliva G, de Cristofaro T, Iuliano L, Brevetti G: Vitamin C prevents endothelial dysfunction induced by acute exercise in patients with intermittent claudication. Atherosclerosis. 2002, 165: 277-283. 10.1016/S0021-9150(02)00235-6. http://www.ncbi.nlm.nih.gov/pubmed/12417278. http://dx.doi.org/10.1016/S0021-9150(02)00235-6
CAS Article PubMed Google Scholar
- 18.
Goldfarb AH, Patrick SW, Bryer S, You T: Vitamin C supplementation affects oxidative-stress blood markers in response to a 30-minute run at 75% VO2max. Int J Sport Nutr Exerc Metab. 2005, 15: 279-290.http://www.ncbi.nlm.nih.gov/pubmed/16131698,
CAS PubMed Google Scholar
- 19.
Shanklin DR, O'Dell TE: Ascorbic acid and the lung. Nature. 1966, 210: 1329-1331. 10.1038/2101329a0.http://dx.doi.org/10.1038/2101329a0,
CAS Article PubMed Google Scholar
- 20.
Castranova V, Wright JR, Colby HD, Miles PR: Ascorbate uptake by isolated rat alveolar macrophages and type II cells. J Applied Physiol. 1983, 54: 208-214. 10.1063/1.331743.http://www.ncbi.nlm.nih.gov/pubmed/6826406,
CAS Article Google Scholar
- 21.
Skoza L, Snyder A, Kikkawa Y: Ascorbic acid in bronchoalveolar wash. Lung. 1983, 161: 99-109. 10.1007/BF02713848. http://www.ncbi.nlm.nih.gov/pubmed/6221162. http://dx.doi.org/10.1007/BF02713848
CAS Article PubMed Google Scholar
- 22.
Slade R, Crissman K, Norwood J, Hatch G: Comparison of antioxidant substances in bronchoalveolar lavage cells and fluid from humans, guinea pigs, and rats. Exp Lung Res. 1993, 19: 469-484. 10.3109/01902149309064358. http://www.ncbi.nlm.nih.gov/pubmed/8370346. http://dx.doi.org/10.3109/01902149309064358
CAS Article PubMed Google Scholar
- 23.
Wiester MJ, Tepper JS, Winsett DW, Crissman KM, Richards JH, Costa DL: Adaptation to ozone in rats and its association with ascorbic acid in the lung. Fundam Appl Toxicol. 1996, 31: 56-64. 10.1006/faat.1996.0075. http://www.ncbi.nlm.nih.gov/pubmed/8998953. http://dx.doi.org/10.1006/faat.1996.0075
CAS Article PubMed Google Scholar
- 24.
Wiester MJ, Winsett DW, Richards JH, Jackson MC, Crissman KM, Costa DL: Ozone adaptation in mice and its association with ascorbic acid in the lung. Inhal Toxicol. 2000, 12: 577-590. 10.1080/08958370050030958. http://www.ncbi.nlm.nih.gov/pubmed/10880145. http://informahealthcare.com/doi/abs/10.1080/08958370050030958
CAS Article PubMed Google Scholar
- 25.
Arad ID, Forman HJ, Fisher AB: Ascorbate efflux from guinea pig and rat lungs: effect of starvation and O2 exposure. J Lab Clin Med. 1980, 96: 673-681.http://www.ncbi.nlm.nih.gov/pubmed/6252268,
CAS PubMed Google Scholar
- 26.
Leung HW, Morrow PE: Interaction of glutathione and ascorbic acid in guinea pig lungs exposed to nitrogen dioxide. Res Commun Chem Pathol Pharmacol. 1981, 31: 111-118.http://www.ncbi.nlm.nih.gov/pubmed/7255866,
CAS PubMed Google Scholar
- 27.
Matzen RN: Effect of vitamin C and hydrocortisone on the pulmonary edema produced by ozone in mice. J Appl Physiol. 1957, 11: 105-109.http://www.ncbi.nlm.nih.gov/pubmed/13462928,
CAS PubMed Google Scholar
- 28.
Mittler S: Protection against death due to ozone poisoning. Nature. 1958, 181: 1063-1064. 10.1038/1811063a0. http://www.ncbi.nlm.nih.gov/pubmed/13541362. http://dx.doi.org/10.1038/1811063a0
CAS Article PubMed Google Scholar
- 29.
Brown LA, Harris FL, Jones DP: Ascorbate deficiency and oxidative stress in the alveolar type II cell. Am J Physiol. 1997, 273: L782-L788. http://www.ncbi.nlm.nih.gov/pubmed/9357853. http://ajplung.physiology.org/content/273/4/L782
CAS PubMed Google Scholar
- 30.
Mudway IS, Stenfors N, Blomberg A, Helleday R, Dunster C, Marklund SL, Frew AJ, Sandström T, Kelly FJ: Differences in basal airway antioxidant concentrations are not predictive of individual responsiveness to ozone. Free Radic Biol Med. 2001, 31: 962-974. 10.1016/S0891-5849(01)00671-2. http://www.ncbi.nlm.nih.gov/pubmed/11595381. http://dx.doi.org/10.1016/S0891-5849(01)00671-2
CAS Article PubMed Google Scholar
- 31.
Glick D, Hosoda S: Ascorbic acid in normal mast cells and macrophages and in neoplastic mast cells. Proc Soc Exp Biol Med. 1965, 119: 52-56. 10.3181/00379727-119-30097.http://www.ncbi.nlm.nih.gov/pubmed/14297882,
CAS Article PubMed Google Scholar
- 32.
Ortner MJ: The oxidation of endogenous ascorbic acid during histamine secretion by rat peritoneal mast cells. Exp Cell Res. 1980, 129: 485-487. 10.1016/0014-4827(80)90521-2.http://dx.doi.org/10.1016/0014-4827(80)90521-2,
CAS Article PubMed Google Scholar
- 33.
Dawson W, West GB: The influence of ascorbic acid on histamine metabolism in guinea-pigs. Br J Pharmacol. 1965, 24: 725-734.http://dx.doi.org/10.1111/j.1476-5381.1965.tb01628.x,
CAS Google Scholar
- 34.
Chatterjee IB, Gupta SD, Majumder AK, Nandi BK, Subramanian N: Effect of ascorbic acid on histamine metabolism in scorbutic guinea-pigs. J Physiol. 1975, 251: 271-279. http://www.ncbi.nlm.nih.gov/pubmed/52707. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1348426
PubMed Central CAS Article PubMed Google Scholar
- 35.
Johnston CS, Huang SN: Effect of ascorbic acid nutriture on blood histamine and neutrophil chemotaxis in guinea pigs. J Nutr. 1991, 121: 126-130.http://jn.nutrition.org/content/121/1/126,
CAS PubMed Google Scholar
- 36.
Nandi BK, Subramanian N, Majumder AK, Chatterjee IB: Effect of ascorbic acid on detoxification of histamine under stress conditions. Biochem Pharmacol. 1974, 23: 643-647. 10.1016/0006-2952(74)90629-7. http://www.ncbi.nlm.nih.gov/pubmed/4132605. http://dx.doi.org/10.1016/0006-2952(74)90629-7
CAS Article PubMed Google Scholar
- 37.
Clemetson CA: Histamine and ascorbic acid in human blood. J Nutr. 1980, 110: 662-668. http://www.ncbi.nlm.nih.gov/pubmed/7365537. http://jn.nutrition.org/content/110/4/662
CAS PubMed Google Scholar
- 38.
Johnston CS, Retrum KR, Srilakshmi JC: Antihistamine effects and complications of supplemental vitamin C. J Am Diet Assoc. 1992, 92: 988-989.http://www.ncbi.nlm.nih.gov/pubmed/1640045,
CAS PubMed Google Scholar
- 39.
Johnston CS, Solomon RE, Corte C: Vitamin C depletion is associated with alterations in blood histamine and plasma free carnitine in adults. J Am Coll Nutr. 1996, 15: 586-591. 10.1080/07315724.1996.10718634. http://www.ncbi.nlm.nih.gov/pubmed/8951736. http://dx.doi.org/10.1080/07315724.1996.10718634
CAS Article PubMed Google Scholar
- 40.
Hagel AF, Layritz CM, Hagel WH, Hagel HJ, Hagel E, Dauth W, Kressel J, Regnet T, Rosenberg A, Neurath MF, Molderings GJ, Raithel M: Intravenous infusion of ascorbic acid decreases serum histamine concentrations in patients with allergic and non-allergic diseases. Naunyn Schmiedebergs Arch Pharmacol. 2013, 386: 789-793. 10.1007/s00210-013-0880-1. http://dx.doi.org/10.1007/s00210-013-0880-1. http://www.ncbi.nlm.nih.gov/pubmed/23666445
CAS Article PubMed Google Scholar
- 41.
Guirgis HM: The regulatory role of vitamin C on the adrenal function and resistance to histamine aerosol in the scorbutic guinea-pig. J Pharm Pharmacol. 1965, 17: 674-675. 10.1111/j.2042-7158.1965.tb07586.x.http://dx.doi.org/10.1111/j.2042-7158.1965.tb07586.x,
CAS Article PubMed Google Scholar
- 42.
Dawson W, Hemsworth BA, Stockham MA: Actions of sodium ascorbate on smooth muscle. Br J Pharmacol Chemother. 1967, 31: 269-275. 10.1111/j.1476-5381.1967.tb01997.x.http://dx.doi.org/10.1111/j.1476-5381.1967.tb01997.x,
PubMed Central CAS Article PubMed Google Scholar
- 43.
Brink C, Ridgway P, Douglas JS: Regulation of guinea pig airways in vivo by endogenous prostaglandins. Pol J Pharmacol Pharm. 1978, 30: 157-166.http://www.ncbi.nlm.nih.gov/pubmed/673926,
CAS PubMed Google Scholar
- 44.
Mohsenin V, Tremml PG, Rothberg KG, Souhrada M, Douglas JS: Airway responsiveness and prostaglandin generation in scorbutic guinea pigs. Prostaglandins Leukot Essent Fatty Acids. 1988, 33: 149-155.http://www.ncbi.nlm.nih.gov/pubmed/3186781,
CAS PubMed Google Scholar
- 45.
Zuskin E, Lewis AJ, Bouhuys A: Inhibition of histamine-induced airway constriction by ascorbic acid. J Allergy Clin Immunol. 1973, 51: 218-226. 10.1016/0091-6749(73)90141-3.http://dx.doi.org/10.1016/0091-6749(73)90141-3,
CAS Article PubMed Google Scholar
- 46.
Sipahi E, Ercan ZS: The mechanism of the relaxing effect of ascorbic acid in guinea pig isolated tracheal muscle. Gen Pharmacol. 1997, 28: 757-760. 10.1016/S0306-3623(96)00277-7.http://dx.doi.org/10.1016/S0306-3623(96)00277-7,
CAS Article PubMed Google Scholar
- 47.
Yeadon M, Wilkinson D, Darley-Usmar V, O'Leary VJ, Payne AN: Mechanisms contributing to ozone-induced bronchial hyperreactivity in guinea-pigs. Pulm Pharmacol. 1992, 5: 39-50. 10.1016/0952-0600(92)90016-A.http://dx.doi.org/10.1016/0952-0600(92)90016-A,
CAS Article PubMed Google Scholar
- 48.
Puglisi L, Berti F, Bosisio E, Longiave D, Nicosia S: Ascorbic acid and PGF2α antagonism on tracheal smooth muscle. Adv Prostaglandin Tromboxane Res. 1976, 1: 503-506.http://www.ncbi.nlm.nih.gov/pubmed/998441,
CAS Google Scholar
- 49.
Rothberg KG, Hitchcock M: Effects of ascorbic acid deficiency on the in vitro biosynthesis of cyclooxygenase metabolites in guinea pig lungs. Prostaglandins Leukot Med. 1983, 12: 137-147. 10.1016/0262-1746(83)90077-X.http://dx.doi.org/10.1016/0262-1746(83)90077-X,
CAS Article PubMed Google Scholar
- 50.
Ogilvy CS, DuBois AB, Douglas JS: Effects of ascorbic acid and indomethacin on the airways of healthy male subjects with and without induced bronchoconstriction. J Allergy Clin Immunol. 1981, 67: 363-369. 10.1016/0091-6749(81)90081-6.http://dx.doi.org/10.1016/0091-6749(81)90081-6,
CAS Article PubMed Google Scholar
- 51.
Tecklenburg SL, Mickleborough TD, Fly AD, Bai Y, Stager JM: Ascorbic acid supplementation attenuates exercise-induced bronchoconstriction in patients with asthma. Respir Med. 2007, 101: 1770-1778. 10.1016/j.rmed.2007.02.014. http://www.ncbi.nlm.nih.gov/pubmed/17412579. http://dx.doi.org/10.1016/j.rmed.2007.02.014
Article PubMed Google Scholar
- 52.
Schock BC, Koostra J, Kwack S, Hackman RM, Van Der Vliet A, Cross CE: Ascorbic acid in nasal and tracheobronchial airway lining fluids. Free Radic Biol Med. 2004, 37: 1393-1401. 10.1016/j.freeradbiomed.2004.07.023. http://www.ncbi.nlm.nih.gov/pubmed/15454278. http://dx.doi.org/10.1016/j.freeradbiomed.2004.07.023
CAS Article PubMed Google Scholar
- 53.
Behndig AF, Blomberg A, Helleday R, Kelly FJ, Mudway IS: Augmentation of respiratory tract lining fluid ascorbate concentrations through supplementation with vitamin C. Inhal Toxicol. 2009, 21: 250-258. 10.1080/08958370802474736.http://dx.doi.org/10.1080/08958370802474736,
CAS Article PubMed Google Scholar
- 54.
Hemilä H: Vitamin C may alleviate exercise-induced bronchoconstriction: a meta-analysis. BMJ Open. 2013, 3: e002416-http://dx.doi.org/10.1136/bmjopen-2012-002416,
PubMed Central Article PubMed Google Scholar
- 55.
Hemilä H: Vitamin C should be tested against exercise-induced bronchoconstriction. Am J Respir Crit Care Med. 2013, 188: 1370-http://dx.doi.org/10.1164/rccm.201307-1205LE,
Article PubMed Google Scholar
- 56.
Schachter EN, Schlesinger A: The attenuation of exercise-induced bronchospasm by ascorbic acid. Ann Allergy. 1982, 49: 146-151.http://www.ncbi.nlm.nih.gov/pubmed/7114587,
CAS PubMed Google Scholar
- 57.
Cohen HA, Neuman I, Nahum H: Blocking effect of vitamin C in exercise-induced asthma. Arch Pediatr Adolescent Med. 1997, 151: 367-370. 10.1001/archpedi.1997.02170410041005.http://dx.doi.org/10.1001/archpedi.1997.02170410041005,
CAS Article Google Scholar
- 58.
Murphy JD, Ferguson CS, Brown KR, Harms CA: The effect of dietary antioxidants on lung function in exercise induced asthma [abstract]. Med Sci Sports Exerc. 2002, 34 (5): S155-
Article Google Scholar
- 59.
McFadden ER: Resurrection men and the FEF25-75. J Allergy Clin Immunol. 2010, 126: 535-536. 10.1016/j.jaci.2010.06.035.http://dx.doi.org/10.1016/j.jaci.2010.06.035,
Article PubMed Google Scholar
- 60.
Simon MR, Chinchilli VM, Phillips BR, Sorkness CA, Lemanske RF, Szefler SJ, Taussig L, Bacharier LB, Morgan W: Forced expiratory flow between 25% and 75% of vital capacity and FEV1/forced vital capacity ratio in relation to clinical and physiological parameters in asthmatic children with normal FEV1 values. J Allergy Clin Immunol. 2010, 126: 527-534. 10.1016/j.jaci.2010.05.016. http://www.ncbi.nlm.nih.gov/pubmed/20638110. http://dx.doi.org/10.1016/j.jaci.2010.05.016
PubMed Central Article PubMed Google Scholar
- 61.
Haas F, Axen K, Schicchi JS: Use of maximum expiratory flow-volume curve parameters in the assessment of exercise-induced bronchospasm. Chest. 1993, 103: 64-68. 10.1378/chest.103.1.64. http://www.ncbi.nlm.nih.gov/pubmed/8417938. http://dx.doi.org/10.1378/chest.103.1.64
CAS Article PubMed Google Scholar
- 62.
Custovic A, Arifhodzic N, Robinson A, Woodcock A: Exercise testing revisited. The response to exercise in normal and atopic children. Chest. 1994, 105: 1127-1132. 10.1378/chest.105.4.1127. http://www.ncbi.nlm.nih.gov/pubmed/8162737. http://dx.doi.org/10.1378/chest.105.4.1127
CAS Article PubMed Google Scholar
- 63.
Fonseca-Guedes CH, Cabral AL, Martins MA: Exercise-induced bronchospasm in children: comparison of FEV1 and FEF25-75% responses. Pediatr Pulmonol. 2003, 36: 49-54. 10.1002/ppul.10309. http://www.ncbi.nlm.nih.gov/pubmed/12772223. http://dx.doi.org/10.1002/ppul.10309
Article PubMed Google Scholar
- 64.
Bar-Yishay E, Amirav I, Goldberg S: Comparison of maximal midexpiratory flow rate and forced expiratory flow at 50% of vital capacity in children. Chest. 2003, 123: 731-735. 10.1378/chest.123.3.731. http://www.ncbi.nlm.nih.gov/pubmed/12628870. http://dx.doi.org/10.1378/chest.123.3.731
Article PubMed Google Scholar
- 65.
Hemilä H: Vitamin C and common cold incidence: a review of studies with subjects under heavy physical stress. Int J Sports Med. 1996, 17: 379-383. 10.1055/s-2007-972864. http://dx.doi.org/10.1055/s-2007-972864. Available at: http://hdl.handle.net/10250/7983
Article PubMed Google Scholar
- 66.
Hemilä H, Chalker E: Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013, 1: CD000980-http://dx.doi.org/10.1002/14651858.CD000980.pub4,
PubMed Google Scholar
- 67.
Peters EM, Goetzsche JM, Grobbelaar B, Noakes TD: Vitamin C supplementation reduces the incidence of postrace symptoms of upper-respiratory-tract infection in ultramarathon runners. Am J Clin Nutr. 1993, 57: 170-174. http://www.ncbi.nlm.nih.gov/pubmed/8185726. http://ajcn.nutrition.org/content/57/2/170
CAS PubMed Google Scholar
- 68.
Peters EM, Goetzsche JM, Joseph LE, Noakes TD: Vitamin C as effective as combinations of anti-oxidant nutrients in reducing symptoms of upper respiratory tract infection in ultramarathon runners. S Afr J Sports Med. 1996, 11: 23-27.
Google Scholar
- 69.
Moolla ME: MSc Thesis. The Effect of Supplemental Anti-Oxidants on the Incidence and Severity of Upper Respiratory Tract Infections in Ultra-Marathon Runners. 1996, South Africa: University of Cape Town
Google Scholar
- 70.
Sabiston BH, Radomski MW: Health Problems and Vitamin C in Canadian Northern Military Operations. DCIEM Report no. 74-R-1012. 1974, Downsview, Ontario, Canada: Defence and Civil Institute of Environmental Medicine, 1-10. Available at: http://www.mv.helsinki.fi/home/hemila/CC/Sabiston_1974_ch.pdf (accessed 18 Nov 2014)
Google Scholar
- 71.
Ritzel G: Critical analysis of the role of vitamin C in the treatment of the common cold [in German]. Helv Med Acta. 1961, 28: 63-68. Translation at: http://www.mv.helsinki.fi/home/hemila/T3.pdf (accessed 18 Nov 2014)
CAS PubMed Google Scholar
- 72.
Kippelen P, Anderson SD: Airway injury during high-level exercise. Br J Sports Med. 2012, 46: 385-390. 10.1136/bjsports-2011-090819.http://dx.doi.org/10.1136/bjsports-2011-090819,
Article PubMed Google Scholar
- 73.
Eccles R: Is the common cold a clinical entity or a cultural concept?. Rhinology. 2013, 51: 3-8.http://www.ncbi.nlm.nih.gov/pubmed/23441305,
CAS PubMed Google Scholar
- 74.
Spence L, Brown WJ, Pyne DB, Nissen MD, Sloots TP, McCormack JG, Locke AS, Fricker PA: Incidence, etiology, and symptomatology of upper respiratory illness in elite athletes. Med Sci Sports Exerc. 2007, 39: 577-586. 10.1249/mss.0b013e31802e851a. http://www.ncbi.nlm.nih.gov/pubmed/17414793. http://dx.doi.org/10.1249/mss.0b013e31802e851a
Article PubMed Google Scholar
- 75.
Arruda E, Pitkäranta A, Witek TJ, Doyle CA, Hayden FG: Frequency and natural history of rhinovirus infections in adults during autumn. J Clin Microbiol. 1997, 35: 2864-2868. http://www.ncbi.nlm.nih.gov/pubmed/9350748. http://jcm.asm.org/content/35/11/2864
PubMed Central CAS PubMed Google Scholar
- 76.
Constantini NW, Dubnov-Raz G, Eyal BB, Berry EM, Cohen AH, Hemilä H: The effect of vitamin C on upper respiratory infections in adolescent swimmers: a randomized trial. Eur J Pediatr. 2011, 170: 59-63. 10.1007/s00431-010-1270-z. http://www.ncbi.nlm.nih.gov/pubmed/20689965. http://dx.doi.org/10.1007/s00431-010-1270-z
CAS Article PubMed Google Scholar
- 77.
Bougault V, Boulet LP: Airway dysfunction in swimmers. Br J Sports Med. 2012, 46: 402-406. 10.1136/bjsports-2011-090821.http://dx.doi.org/10.1136/bjsports-2011-090821,
Article PubMed Google Scholar
- 78.
Wenzel SE: Asthma: defining of the persistent adult phenotypes. Lancet. 2006, 368: 804-813. 10.1016/S0140-6736(06)69290-8. http://www.ncbi.nlm.nih.gov/pubmed/16935691. http://dx.doi.org/10.1016/S0140-6736(06)69290-8
CAS Article PubMed Google Scholar
- 79.
Lötvall J, Akdis CA, Bacharier LB, Bjermer L, Casale TB, Custovic A, Lemanske RF, Wardlaw AJ, Wenzel SE, Greenberger PA: Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011, 127: 355-360. 10.1016/j.jaci.2010.11.037.http://dx.doi.org/10.1016/j.jaci.2010.11.037,
Article PubMed Google Scholar
- 80.
Akaike T: Role of free radicals in viral pathogenesis and mutation. Rev Med Virol. 2001, 11: 87-101. 10.1002/rmv.303.http://dx.doi.org/10.1002/rmv.303,
CAS Article PubMed Google Scholar
- 81.
Castro SM, Guerrero-Plata A, Suarez-Real G, Adegboyega PA, Colasurdo GN, Khan AM, Garofalo RP, Casola A: Antioxidant treatment ameliorates respiratory syncytial virus-induced disease and lung inflammation. Am J Respir Crit Care Med. 2006, 174: 1361-1369. 10.1164/rccm.200603-319OC. http://www.ncbi.nlm.nih.gov/pubmed/17008643. http://dx.doi.org/10.1164/rccm.200603-319OC
PubMed Central CAS Article PubMed Google Scholar
- 82.
Hemilä H: Vitamin C and common cold-induced asthma: a systematic review and statistical analysis. Allergy Asthma Clin Immunol. 2013, 9: 46-10.1186/1710-1492-9-46.http://dx.doi.org/10.1186/1710-1492-9-46,
PubMed Central Article PubMed Google Scholar
- 83.
Fogarty A, Lewis SA, Scrivener SL, Antoniak M, Pacey S, Pringle M, Britton J: Oral magnesium and vitamin C supplements in asthma: a parallel group randomized placebo-controlled trial. Clin Exp Allergy. 2003, 33: 1355-1359. 10.1046/j.1365-2222.2003.01777.x. http://www.ncbi.nlm.nih.gov/pubmed/14519140. http://dx.doi.org/10.1046/j.1365-2222.2003.01777.x
CAS Article PubMed Google Scholar
- 84.
Heart Protection Study Collaborative Group: MRC/BHF heart protection study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002, 360: 23-33. http://www.ncbi.nlm.nih.gov/pubmed/12114037. http://dx.doi.org/10.1016/S0140-6736(02)09328-5
Article Google Scholar
- 85.
Fogarty A, Lewis SA, Scrivener SL, Antoniak M, Pacey S, Pringle M, Britton J: Corticosteroid sparing effects of vitamin C and magnesium in asthma: a randomised trial. Respir Med. 2006, 100: 174-179. 10.1016/j.rmed.2005.03.038. http://www.ncbi.nlm.nih.gov/pubmed/16338599. http://dx.doi.org/10.1016/j.rmed.2005.03.038
Article PubMed Google Scholar
- 86.
Goodwin JS, Tangum MR: Battling quackery: attitudes about micronutrient supplements in American Academic medicine. Arch Intern Med. 1998, 158: 2187-2191. 10.1001/archinte.158.20.2187.http://dx.doi.org/10.1001/archinte.158.20.2187,
CAS Article PubMed Google Scholar
- 87.
Richards E: The politics of therapeutic evaluation: the vitamin C and cancer controversy. Soc Stud Sci. 1988, 18: 653-701. 10.1177/030631288018004004.http://dx.doi.org/10.1177/030631288018004004,
Article Google Scholar
- 88.
Louhiala P, Hemilä H: Can CAM treatments be evidence-based?. Focus Altern Complement Ther. 2014, 19: 84-89. 10.1111/fct.12110.http://dx.doi.org/10.1111/fct.12110,
Article Google Scholar
- 89.
Hemilä H, Herman ZS: Vitamin C and the common cold: a retrospective analysis of Chalmers' review. J Am Coll Nutr. 1995, 4: 116-123. http://dx.doi.org/10.1080/07315724.1995.10718483. Available at: http://hdl.handle.net/10138/42358
Article Google Scholar
- 90.
Hemilä H: Vitamin C supplementation and common cold symptoms: problems with inaccurate reviews. Nutrition. 1996, 12: 804-809. 10.1016/S0899-9007(96)00223-7. http://dx.doi.org/10.1016/S0899-9007(96)00223-7. Available at: http://hdl.handle.net/10250/7979
Article PubMed Google Scholar
- 91.
Kaur B, Rowe BH, Arnold E: Vitamin C supplementation for asthma. Cochrane Database Syst Rev. 2009, 1: CD000993-http://dx.doi.org/10.1002/14651858.CD000993.pub3,
PubMed Google Scholar
- 92.
Hemilä H: Feedback to Cochrane review on vitamin C and asthma. 2009,http://hdl.handle.net/10138/38500,
Google Scholar
- 93.
Hemilä H: Vitamin C and exercise-induced bronchoconstriction: further problems in the Cochrane review "vitamin C for asthma". 2013,http://hdl.handle.net/10138/40816,
Google Scholar
Download references
Author information
Affiliations
-
Department of Public Health, POB 41 University of Helsinki, Mannerheimintie 172, FIN-00014, Helsinki, Finland
Harri Hemilä
Corresponding author
Correspondence to Harri Hemilä.
Additional information
Competing interests
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The author declares that he has no competing interests.
Electronic supplementary material
Authors' original submitted files for images
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Reprints and Permissions
About this article
Cite this article
Hemilä, H. The effect of vitamin C on bronchoconstriction and respiratory symptoms caused by exercise: a review and statistical analysis. All Asth Clin Immun 10, 58 (2014). https://doi.org/10.1186/1710-1492-10-58
Download citation
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/1710-1492-10-58
Keywords
- Anti-asthmatic agents
- Ascorbic acid
- Cough
- Histamine
- Exercise-induced asthma
- Forced expiratory flow rates
- Meta-analysis
- Prostaglandin
- Randomized controlled trial
- The lungs
Is Vitamin C Good For The Lungs
Source: https://aacijournal.biomedcentral.com/articles/10.1186/1710-1492-10-58
- Dapatkan link
- X
- Aplikasi Lainnya
